Ultrastructural observations using the field emission microscope and transmission electron microscope on a Silurian dendroid from Gothland (Sweden)
Denis
Edwin Beeching Bates1
1Institute of Geography and Earth Sciences, University of Wales Aberystwyth, Aberystwyth, Ceredigion SY23 3QQ, United Kingdom. E–mail: deb@aber.ac.uk
Key words: Dendrograptus. Ultrastructure. Cortex. Fusellum. Stolon.
Introduction
In 1997 the author described the ultrastructure of ?Dendrograptus from the Silurian of Gothland, Sweden (Bates 1997), using both the Scanning Electron Microscope (SEM) and the Transmission Electron Microscope (TEM). Since then, it has become possible to examine the same material using the Field Emission Microscope (FEM), which gives a much better resolution than the ordinary SEM. As a result, further detail has been observed, and some amendments can also be made to the results presented in the earlier paper.
Material and locality
The material comes from the topmost Lower Visby Formation, 5.32–5.44 metres above the base of the Lusklint Bentonite, Ireviken, Gothland, Sweden. Upper Llandovery: P. amorphognathoides Zone (Lower P. procerus Zone of Jeppsson, 1994). Specimens on SEM stubs have been deposited in the collections of the Department of Historical Geology and Palaeontology, University of Lund, Sweden.
The material consists only of portions of stipes, which have dichotomous branching. The stipes are straight, in young specimens with denticulate autothecae clearly visible, in old specimens with cortical thickening smothering the thecae to make the stipe cylindrical. The material was prepared by fracturing small pieces of stipe, and mounting them with the stipe axis both parallel and perpendicular to the stub surface. A fine coating of gold–palladium was applied.
Fusellar fabric
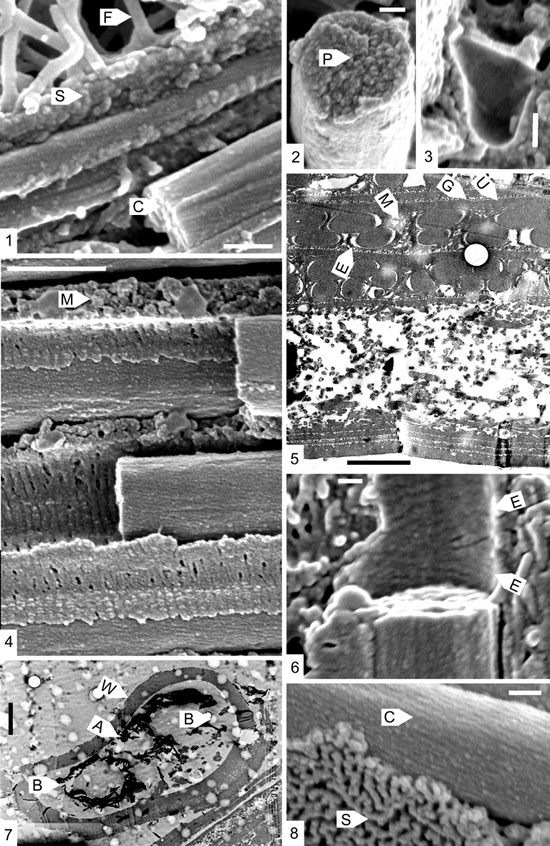
As observed with the SEM (Bates, 1997; Plate 2, Figure 3; Plate 3, Figure 1), the fusellar fabric is composed of smooth–sided fibrils about 50nm thick, and, towards the arch of each fusellus, thicker parallel fibrils. Under the FEM (Figure 1.1) and TEM (Figure 1.5) these parallel fibrils can also be seen to be about 200nm in diameter, and also to have the same clockwise spiralling striations as those of the cortical fibrils described below. They are not, however, surrounded by a sheath of concentric fibrils.

Figure 1. 1. The boundary region between two fuselli. Thick parallel fibrils, with clockwise spiralling striations (C) lie beneath the sheet (S) bounding the first fusellus. At the base of the second fusellus are much thinner fusellar fibrils (F). Scalebar 200nm. 2. Fractured cortical fibril showing the granular nature of the break, with a possible central pore (P). Scalebar100nm. 3. Vesicle within a cortical unit, with a smooth wall. Scalebar 100nm. 4. Portion of cortical unit, showing cortical fibrils, each surrounded by a sheath (E) of concentric fibrils, and an irregular matrix (M). Scalebar 1 mm. 5. TEM micrograph of a cross–section of fusellum and two cortical units. E: sheath of concentric fibrils; M: matrix of unit; G: granular layer of sheet fabric; U: uniform layer of sheet fabric. Scalebar 500nm. 6. Portion of sheath of concentric fibrils surrounding a cortical fibril. Note the two opposed directions of spiralling (E) within the sheath. Scalebar 100nm. 7. TEM micrograph of a transverse section of a stolon. W: stolon wall, of crassal fabric. A: smaller autothecal ring. B: larger bithecal and “stolothecal” rings. Scalebar 2 mm. 8. Cortical fibrils (C) with clockwise spiralling striations, and sheet fabric (S) bounding the cortical unit. Scalebar 100nm.
Sheet fabric
Under the FEM the sheet fabric of the cortical units has a distinctive texture: granules about 25 nm in diameter are grouped into meandering lines (Figure 1.8), or closely packed (Figure 1.1). Under the TEM (Figure1.5; Bates, 1997, Plate 4, Figure 7) the granular layer (G) lies outside a dense and uniform layer (U), which is not so easily observed under the FEM. The sheet fabric of the fuselli is also finely granular (Figure 1.1). It is possible that the granular layer actually forms the basal layer of each cortical unit, the uniform layer the bounding layer of the preceding unit.
Cortical fibrils
Under the SEM, the cortical fibrils appear to be smooth (Bates, 1997, Plate 3, Figure 5), up to about 500nm in diameter. However, under the FEM they can clearly be seen to be finely striated (Figures 1.1, 1.4, 1.6, 1.8). The striations run at an angle of about 20o to the axis of the fibrils, and spiral uniformly in a clockwise direction when viewed along the axis (Figure 1.8). The striations number about ten in 400 nm. Although in TEM the fibrils appear to have a very fine and uniform internal structure, with a translucent core (Bates 1997, Plate 4, Figure 7), in fractured sections the fibrils appear to be coarsely granular, without any clear sign of the core (Figure 1.2). This helical structure is similar to that deduced by Rickards and Dumican (1984, Figure 8) for the fibrils in the virgular fabric in Pristiograptus dubius and Holoretiolites mancki.
Concentric fibrils
The cortical fibrils are surrounded by a sleeve of concentric fibrils, about 45 nm thick (Figures1.4–1.6). These are, as preserved, separated from the cortical fibrils by a space, which is prominent in the TEM sections cut perpendicular to the fibrils (Figure 5). It is possible that these fibrils may spiral round the cortical fibrils, as they can appear to be making an angle of less than 90o to their long axis (Figure 1.6). In the example shown in Figure1.6, there is also the possibility that the spiralling is in two different directions.
Given the higher magnification possible with the FEM, it can be seen that the author’s interpretation of the annulations seen in TEM sections (Bates, 1997, Plate 4, Figure 6), is incorrect. The annulations were there regarded as being within the cortical fibrils, and only visible when they were seen in very oblique section. Re–examination of these micrographs in fact shows that the annulations are in fact the concentric fibrils of the sleeves surrounding the cortical fibrils, and not in the fibrils themselves.
The size of the concentric fibrils compares well with the annulations illustrated by Crowther and Rickards (1977, Plate 3, Figures 1–2) and Crowther (1981, Plate 2, Figure 2) in Dictyonema rarum Wiman. However, their annulations appear to be a corrugation on the surface of the cortical fibrils, not a separate structure. It may be that in Dendrograptus this separation between the concentric fibrils and the cortical fibrils is a post mortem effect: in life they may have closely adhered to the surface of the cortical fibrils.
Cortical matrix
The FEM (Figure 1.4) has not shown any more detail of the matrix of the cortical units than that revealed by the SEM. It still appears to have a spongy texture, without any fibrillar component as observed in TEM section (Figure 1.5).
Vesicles
The vesicles, which occur both immediately beneath sheet fabrics, and within the matrix, can be seen to have extremely smooth walls (Figure 1.3). The small globular bodies, which are occasionally seen within them, have equally smooth surfaces.
Stolon
The stolon was not observed in any of the fractured pieces of stipe, but was present in one of the sections made for the TEM (Figure 1.7). Although slightly fractured, the stolon appears to have been oval in section, with a wall about 1 mm thick, made of a dense crassal fabric showing no internal structure. In the interior are three roughly circular rings, made of dense fibrils or spicules, a smaller central one (A: about 2 mm in diameter) flanked by two larger ones (B: between 3–4 mm in diameter). These rings appear similar in position and size to the arrangement of pores leading into the proximal side of the stolonal node of a dendroid stolon, as illustrated by Crowther (1981; Plate 4, Figure 3). Hence they can be interpreted as forming three canals at the proximal side of a node, each passing into one of the chambers of the node. The smaller pore corresponds to the autothecal stolon on the distal side of the node, and the two larger pores to the bithecal and succeeding "stolothecal" stolon in a normal node.
Discussion
The main conclusion of the study is that the make–up of the cortical units is more complex than has been hitherto recognised. Each unit appears to be composed of at least five different components: sheet fabric (itself here described as being of two layers), cortical matrix, cortical fabric, concentric fabric and vesicles. The assembly formed by the sheath of concentric fabric, laid down round the thick and spiralling cortical fibrils, is of particular interest from a structural point of view. It appears to be analogous to some cable structures, where a major inner cable, made with spiralling wires, is encased in an outer sheath. Clearly the major cortical fibrils give the main strength and resilience to the whole rhabdosome: a purpose which is particularly well demonstrated in structures such as spines like the graptoloid nema, or lists as in retiolite graptolites.
The spicular rings found within the stolon suggest that its internal structure is more complex than hitherto realised. The dendroid stolon forms as a continuous series of segments (each within a "stolotheca"), each of circular cross–section, but widening to an oval section just before branching at a node. The three ring structures indicate that branching of the interior of the stolon into three canals would have already taken place before the development of the node. The spicules appear unlike any other material observed, and it is even possible that they are the degraded remnants of soft tissue.
Acknowledgements
I am grateful to Dr. Lennart Jeppsson, of the University of Lund, Sweden, who provided the material, from collections made by him, and also led a field excursion to the locality, providing hospitality at the Lund University field station, Allekvia. Mr. Phillip Lloyd, of the Institute of Biology, University of Wales, Aberystwyth, prepared the TEM sections. I would like to thank Mr. Stephen Wade, also of the Institute of Biology, for both technical help with the FEM examination, and discussion of the material. The work was carried out using a grant from the Natural Environment Research Council (UK).
References
Bates, D.E.B. 1997. The ultrastructure of a Silurian dendroid from Gotland (Sweden). Geobios, 20: 27–37.
Crowther, P. 1981. The fine structure of graptolite periderm. Special Papers in Palaeontology, 26: 1–119.
Crowther, P. and Rickards, R.B. 1977. Cortical bandages and the graptolite zooid. Geologica Palaont., 11: 9–46.
Rickards, R.B. and Dumican, L.W. 1984. The fibrillar component of the graptolite periderm. Irish Journ. Earth Science, 6: 175–203.
Received: February 15, 2003
Accepted: June 15, 2003