Paleoecological significance of the Ibexian–Whiterockian (Lower–Middle Ordovician) boundary in the Great Basin, Western US
Seth Finnegan1 and Mary L. Droser1
1 Department of Earth Sciences, University of California – Riverside, CA 92521. E–mail: sfinn@citrus.ucr.edu
Key words: Paleoecology. Diversification. Ibexian–Whiterockian boundary. Great Basin. USA.
Introduction
The Great Basin of Utah, Nevada, and California has been an important region for paleontological and stratigraphic research on the Ordovician System for more than 100 years. The North American type sections of the Lower Ordovician (Ibexian) and Middle Ordovician (Whiterockian) are in the region, and sections are commonly accessible, well–exposed, and richly fossiliferous. Ibexian strata record a broad, shallow, mixed carbonate–clastic ramp lying on or near the equator, which developed into a rimmed platform in the Whiterockian (Ross et al., 1989). Despite complex lateral and vertical lithological changes reflecting the interplay of sea–level change, subsidence and local tectonics, the region is characterized by general environmental stability throughout the Lower and lower Middle Ordovician (Ross, 1977; Ross et al., 1989; Ross et al., 1997), and there are no major unconformities (Ross et al., 1991, 1997). Though it has long been a focus of taxonomic and biostratigraphic research, only relatively recently have explicitly paleoecological studies been undertaken in the Great Basin. As a consequence, our knowledge of the ecological context of diversification in the Great Basin lags considerably our knowledge of the pattern of diversification. Despite this general uncertainty, a growing body of evidence suggests that the period surrounding the Ibexian–Whiterockian boundary is a critical interval in the reorganization of Great Basin paleocommunities (Berry, 1974; Droser et al., 1996).
Global patterns
Taxonomically, the Ibexian–Whiterockian boundary is of global significance. Miller and Foote’s (1996) rarefaction analysis of global genus diversity confirmed the major mid–Arenig jump in standing diversity initially identified by Sepkoski (1995), which accounts for a large part of the total Ordovician diversity increase. Their data also show a trend in trilobite disparity which closely parallels the global genus diversity curve, demonstrating that within the Trilobita, at least, diversification was morphological as well as taxonomic. Adrain et al. (1998) showed that the trilobite families which diversify at this time (following, in most cases, earlier origins) are those which account for all post–Ordovician trilobite diversity. Rhynchonelliform brachiopods (especially the Orthida and Strophomenida) also diversify at this time, as do corals, bryozoans, and echinoderms (Guensberg and Sprinkle, 1992; Patzkowsky, 1995; Sepkoski, 1995; Harper and MacNiocaill, 2002).
Local patterns from published faunal lists
An understanding of the dynamics of paleoecological change is most likely to emerge from analyses at the basinal scale, as it is at this scale that the species pools from which communities are assembled are delineated. We thus focus here on broad patterns of faunal turnover through the Ibexian–Whiterockian boundary interval in the Great Basin. The data presented here come in part from a growing database of Great Basin Ordovician faunal lists culled from 20 published sources and supplemented by our own collections. The database includes only whole macrofaunal collections from single beds. Currently the database includes ~350 faunal lists with species richness ranging from 4 (lists of fewer than 4 species are not included) to 24. Trilobites, brachiopods, mollusks, ostracodes, bryozoans, corals, and sponges in 372 genera, 148 families, and 44 orders are represented. Echinoderms are excluded because they are rarely and inconsistently identified, and graptolites because of their planktonic lifestyle and strongly facies–controlled preservation.

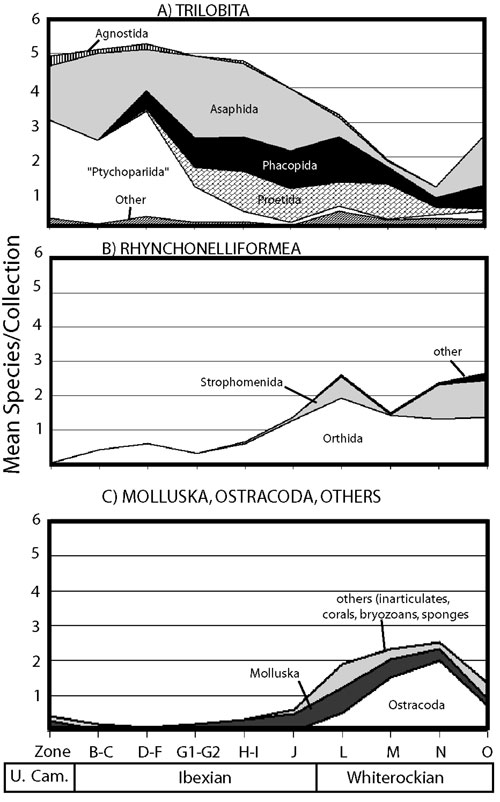
Figure 1. Mean per–collection species diversity for a variety of taxa within 10 Late Cambrian–Middle Ordovician time slices. Because many collections are clearly undersampled, the mean species/collection will almost always be an underestimate of "true" a diversity, but the proportions of taxa should not be strongly affected by this bias.
Because collections come from single beds, the diversity trends presented are at the local, or within–paleocommunity level. However, because they are presented for the Great Basin region as a whole and are averaged across a range of environments in each time–slice, they mask significant geographic patterns of faunal differentiation and represent only very broad basin–wide patterns of faunal occurrence. The data do not represent standing diversity, but rather are an estimate of the ecological significance of a group, as measured by frequency of occurrence in collections.
Trilobita exhibit a pattern of apparently declining local diversity through the Arenig and Llanvirn, but the proportional change in taxonomic dominance among trilobite orders is relatively small (Figure 1A). Asaphida (in particular, the Asaphidae) decline in diversity across the boundary, and account for only a small fraction of trilobite species encounters in Whiterockian collections, though they "rebound" somewhat in the latest Whiterockian due to inclusion of deep–water collections with common Remopleurids and Trinucleids. Proetida and Phacopida (represented primarily by Bathyuridae and Pliomeridae, respectively) are commonly encountered in post–Tremadoc collections, and maintain their proportional significance through the boundary. The groups which Adrain et al., identified as having significant Whiterockian radiations are for the most part rarely encountered in Great Basin collections. While some of these have their first Great Basin appearances at or near the boundary (Fortey and Droser, 1996, 1999) they remain for the most part ecologically minor throughout the Whiterockian series.
The apparent decline in trilobite a diversity should be viewed with some suspicion. The mean whole–fauna richness of collections shows no significant change throughout the Upper Cambrian, Ibexian, and Whiterockian, despite the obvious diversification of several groups (Figure 2B,C). This suggests that the interval may be generally undersampled, and that apparent "richness" patterns are controlled more strongly by sampling intensity than by true diversity trends. This impression is strengthened by examining a histogram of richness for all collections in the database (Figure 2). This is not surprising, given that the vast majority of the collections were made in the service of biostratigraphic studies, not biodiversity studies. Thus, the declining local diversity of trilobites, which is closely complemented by increasing local diversity of rhychonelliform brachiopods, ostracodes, corals, and bryozoans likely represents at least in part the taxonomic dilution (sensu Westrop et al., 1995) of trilobites by an influx of new taxa, rather than a true ecological displacement.
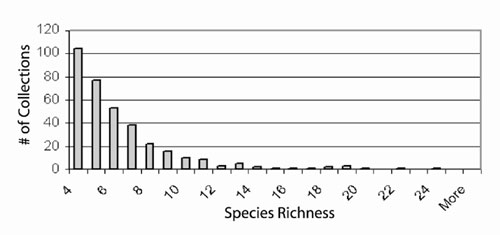
Figure 2. Histogram of species richness for all faunal lists in the database. The lack of an emergent modal richness greater than 4 strongly implies that most collections are undersampled and do not accurately represent true á diversity patterns.
A second complication arises from preservational biases. While both the Ibexian and Whiterockian Series commonly contain silicified faunas, there is an abrupt shift in the taxonomic composition of these faunas between the Ibexian and Whiterockian. In Ibexian strata trilobites are often beautifully silicified, while co–occuring brachiopods are rarely if ever silicified; in Whiterockian strata the reverse is usually true. The apparent changes in trilobite and brachiopod diversity are therefore likely in part due to biases of silicification. Ongoing field work aims to address this issue by using standardized "crack–out" samples.
Local patterns from fieldwork
There is some less equivocal evidence that in the Great Basin trilobites may decline in ecological significance, if not diversity, across the Ibexian–Whiterockian boundary. Li and Droser (1999) reported a sharp transition in the composition of shell beds. While most Ibexian shell beds are trilobite–only or trilobite–dominated, the majority of Whiterockian shell beds are brachiopod–dominated (there are also significant numbers of ostracod–dominated beds). The general pattern in shell bed composition is quite similar to the taxonomic occurrence data presented above. Because of their obvious taphonomic complications, interpretation of the shell bed record is not straightforward (Li and Droser, 1999; Adrain et al., 2000). Less bioclast–rich lithologies present fewer complications and should not be as sensitive to "taphonomic dilution". A more recent study of wackestones across the Ibexian Whiterockian boundary at the classic section J in the Ibex area, UT found a sharp decline in the abundance of trilobite material and a nearly coincident increase in the abundance of Orthid brachiopod material (Figure 3, Finnegan, unpublished). Interestingly, there is very little change in the a diversity of either trilobites or brachiopods across the boundary, suggesting that locally at least diversity and abundance patterns may not be closely coupled. In this and other sections in the area, a significant decline in mean sampled evenness (equitability of distribution of individuals among taxa) of whole–fauna collections occurs across the boundary (Finnegan, unpublished). This pattern is driven largely by the great abundance of the orthid genus Paralenorthis in shallow subtidal settings of zone L (lowermost Whiterockian). Though there is insufficient data to generalize, it appears that the equitability of trilobite faunas considered alone is little affected. In general, the low a diversity of brachiopods in Great Basin Middle Ordovician sections relative to many other regions contrasts with their overwhelming numerical dominance –again implying that taxonomic and ecological dominance, though closely linked, are not interchangeable qualities. However, in the absence of more standardized collections including abundance data it is impossible to test this proposition.
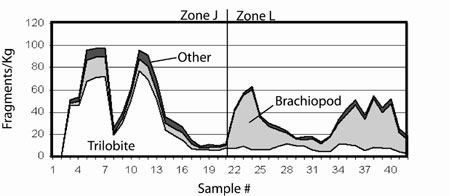
Figure 3. 3–per moving average of shell material abundance and type from 42 wackestone samples collected through 124 meters of section across the Ibexian–Whiterockian boundary (between zones J and L) at Ibex, UT. Though original collections are much larger, shell material concentrations are standardized to 1 kg. Average sample interval is 2.7 meters.
Conclusions
The published literature is of great use in delineating general patterns of taxonomic turnover through the Ordovician biodiversification. However, a refined understanding of the ecological dynamics of Ordovician diversification can only come from a program of detailed regional paleoecological field studies. Collections must be made in a variety of depositional settings using standardized methodologies in order to obtain the diversity–abundance data that is essential for characterizing the shifting ecological (as opposed to taxonomic) structure of paleocommunities. We have begun such a program in the Ibexian and Whiterockian strata of the Great Basin. Preliminary results indicate that a significant ecological restructuring took place across the boundary interval, but the nature of the transition in poorly understood. Future work will focus on delineating the trajectory of ecological change (through the diversification period in general and across the boundary in particular), as well as on the spatial diversity accommodation patterns (a vs. b diversity), and possible temporal and trends in sampled evenness.
References
Adrain, J.M., Fortey, R.A. and Westrop, S.R. 1998. Post–Cambrian trilobite diversity and evolutionary faunas. Science, 280: 1922–1925.
Adrain, J.M., Westrop, S.R., Chatterton, B.D.E. and Ramskold, L. 2000. Silurian trilobite alpha diversity and the end–Ordovician mass extinction. Paleobiology, 26: 625–646.
Berry, W.B.N. 1974. Types of ealy Paleozoic faunal replacements in North America: their relationship to environmental change. Journal of Geology, 82: 371–382.
Droser, M.L., Fortey, R.A. and Li, X. 1996. The Ordovician radiation, American Scientist, 84: 122.
Fortey, R.A. and Droser, M.L. 1996. Trilobites at the base of the middle Ordovician, Western United States, Journal of Paleontology, 70: 73–99.
Fortey, R.A. and Droser, M.L. 1999. Trilobites from the base of the type Whiterockian, Journal of Paleontology, 73: 182–201.
Guensberg, T.E. and Sprinkle, J. 1992. Rise of echinoderms in the Paleozoic evolutionary fauna; significance of paleoenvironmental controls. Geology, 20: 407–410.
Harper, D.A.T. and MacNiocaill, C. 2002. Early Ordovician brachiopod biodiversity: comparing some platforms, margins, and intra–oceanic sites around the Iapetus ocean. In: Crame, J.A., and Owen, A.W., (Ed.), Paleobiogeography and Biodiversity Change: the Ordovician and Mesozoic–Cenozoic Radiations. Geological Society, London, Special Publication, 194: 25–34
Li, X., Droser, M.L. 1999. Lower and Middle Ordovician shell beds from the Basin and Range province of the western United States (California, Nevada, and Utah). Palaios, 14: 215–233.
Miller, A.I., Foote, M. 1996. Calibrating the Ordovician radiation of marine life – implications for Phanerozoic diversity trends. Paleobiology, 22: 304–309.
Patzkowsky, M.E. 1995. Ecologic aspects of the Ordovician radiation of articulate brachiopods. In: Cooper, J.D., Droser, M.L., and Finney, S.C., (Ed.), Ordovician odyssey: short papers for the seventh international symposium on the Ordovician System. Pacific Section SEPM. 413–414.
Ross, R.J., Jr. 1977. Ordovician Paleogeography of the western United States. In: Stewart, J.H., Stevens, C.H., and Fritsche, A.E., (Ed.), Pacific coast paleogeography symposium 1, Paleozoic paleogeography of the western United States. Pacific Section, Society of Economic Paleontologists and Mineralogists. 19–38.
Ross, R.J., Jr., James, N.P., Hintze, L.F., and Poole, F.G. 1989. Architecture and evolution of a Whiterockian (early Middle Ordovician) carbonate platform, Basin Ranges of western U.S.A. In: Crevallo, P.D., Wilson, J.L., Sarg, J.F., and Read, J.F., (Ed.), Controls on carbonate platform and basin development, 44. SEPM Special Publication. 167–185.
Ross, R.J., Jr., and Ethington, R.L. 1991. Stratotype of Ordovician Whiterock Series. Palaios, 6: 156–173
Ross, R.J., Jr., Hintze, L.H., Ethington, R.L., Miller, J.F., Taylor, M.E, and Repetski, J.E. 1997. The Ibexian, lowermost series in the North American Ordovician, with a section on echinoderm biostratigraphy by Sprinkle, J., and Guensberg, T.E. In: Taylor, M.E., (Ed.), Early Paleozoic biochronology of the Great Basin, western United States. U.S. Geological Survey Professional Paper 1579: 1–50.
Sepkoski, J.J., Jr. 1995. The Ordovician radiations: diversification and extinction shown by global genus–level data. In: Cooper, J.D., Droser, M.L., and Finney, S.C., (Ed.), Ordovician odyssey: short papers for the seventh international symposium on the Ordovician System. Pacific Section SEPM: 393–396.
Westrop, S.R., Tremblay, J.V., Landing, E. 1995. Declining Importance of Trilobites in Ordovician Nearshore Paleocommunities – Dilution or Displacement. Palaios, 10: 75–79.
Received: February 15, 2003
Accepted: June 15, 2003